Xiaokun Zhang | Molecular Cell: Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy
Highlights
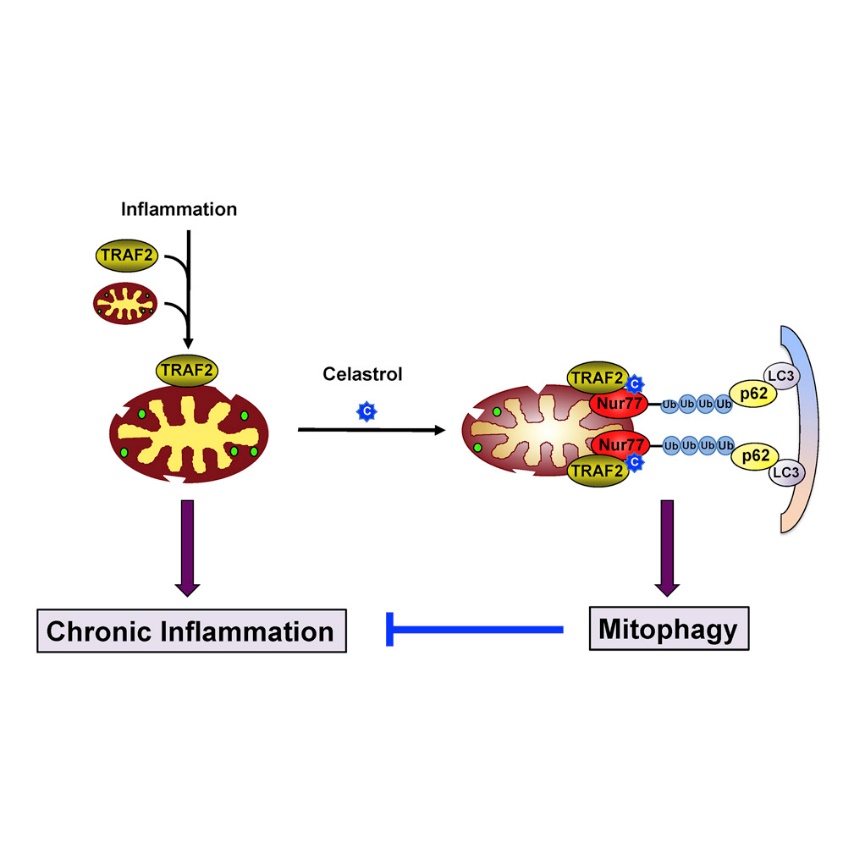
Celastrol binds Nur77 to inhibit inflammation by autophagy
Celastrol induces Nur77 translocation to mitochondria and interaction with TRAF2
An LxxLL motif in TRAF2 mediates its interaction with Nur77
Celastrol promotes Nur77 ubiquitination, p62/SQSTM1 interaction, and mitophagy
Summary
Mitochondria play an integral role in cell death, autophagy, immunity, and inflammation. We previously showed that Nur77, an orphan nuclear receptor, induces apoptosis by targeting mitochondria. Here, we report that celastrol, a potent anti-inflammatory pentacyclic triterpene, binds Nur77 to inhibit inflammation and induce autophagy in a Nur77-dependent manner. Celastrol promotes Nur77 translocation from the nucleus to mitochondria, where it interacts with tumor necrosis factor receptor-associated factor 2 (TRAF2), a scaffold protein and E3 ubiquitin ligase important for inflammatory signaling. The interaction is mediated by an LxxLL motif in TRAF2 and results not only in the inhibition of TRAF2 ubiquitination but also in Lys63-linked Nur77 ubiquitination. Under inflammatory conditions, ubiquitinated Nur77 resides at mitochondria, rendering them sensitive to autophagy, an event involving Nur77 interaction with p62/SQSTM1. Together, our results identify Nur77 as a critical intracellular target for celastrol and unravel a mechanism of Nur77-dependent clearance of inflamed mitochondria to alleviate inflammation.
Link: https://www.sciencedirect.com/science/article/pii/S1097276517301983